Bcl3 Molecular Shape
This is because the molecular arrangement of the chlorine atom is part of a complete triangular shape. BCl 3 consists of one Boron atom and three Chlorine atoms.

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
1 The Molecular Geometry Of The BCl3 Molecule Is And This Molecule Is - A Trigonal Pyramidal.
Bcl3 molecular shape. Chemistry questions and answers. Valence of e- on central atom ie. A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure Boron trichlorideFor the BCl3 structure use the periodic table to find the total nu.
An explanation of the molecular geometry for the BCl3 ion Boron trichloride including a description of the BCl3 bond angles. For the above molecule VSEPR notation will be AX 3 E 0. This record has not been tagged.
The electron geometry for the. The bond angle is 120o. BCl 3 Molecular Geometry And Bond Angles.
Become a member and unlock all Study. Therefore there exists no polarization of charges across the BCl3 molecule. A Lewis structure is first structure and has two extra lone pairs on the central atom.
Read More About Hybridization of Other Chemical Compounds. The molecular geometry of the BCl3 molecule is _____ and this molecule is _____. The molecular geometry of BCl3 is trigonal pyramidal.
For more information you must also go through an article on the polarity of BCl3. Use lewis structure guidelines to draw the lewis structure of BCl 3. If we look at the structure BCl 3 molecular geometry is trigonal planar.
Draw its VSEPR and Lewis structure. The central atom also has a symmetric charge around it and the molecule is non-polar. Youll find the correct answer below.
The BCl3 molecule is considered to be non-polar because the charge distribution across the molecule is uniform as the shape of the molecule is symmetric ie. BCl3 takes the shape of trigonal planar. XNumber of surrounding atoms.
Both BCl3 and ICl3 have 3 bonds. C Molecular shape is T shaped. BCl 3 has a.
All the three bond angles in boron trichloride BCl3 B C l 3 are equal and equal to 120. The bond angles and molecular shape of. Average mass 117170 Da.
A trigonal pyramidal polar B trigonal pyramidal nonpolar C trigonal planar polar D trigonal planar nonpolar E trigonal bipyramidal polar. Which of the molecules has a see-saw shape. Apply VSEPR notation A X E.
Here is the answer for the question The molecular geometry of the BCl3 molecule is _____ and this molecule is _____. BCl 3 has an sp 2 hybridization state. Molecular geometry is trigonal planar.
B VSEPR 3 bp 2 lp 5 shape is trigonal bipyramidal. Chemistry Molecular Orbital Theory Molecular Geometry. BeCl2 has minimum energy when it is a linear molecule.
1 The Molecular Geometry Of The BCl3 Molecule Is And This Molecule Is - A Trigonal Pyramidal Polar B Trigonal Pyramidal Nonpolar C Trigonal Planar Polar D Trigonal Planar Nonpolar E Trigonal Bipyramidal Polar 2 Which One Of The Following Is. The bond angle is 120 o. How do I determine the bond angle in a molecule.
If we look at the structure BC l3. Use VSEPR table to find the shape. In its most stable state Boron forms three covalent bonds with the surrounding Chlorine atoms making for three bonded pairs in the centre.
Boron are 3 no. The boron is located in the center which has three valence electrons and balances out the three chlorine. Because of similar vapor pressure - temperature curves BCl3 and COCl2 cannot be separated by fractional distillation.
1 Answer Humaam H. Jul 12 2014 Answer link. Describe the hybridization electron geometry molecular geometry and polarity for each and discuss similarities and differences.
The molecular geometry of the BCl3 molecule is _____ and this molecule is _____-trigonal pyramidal polar-trigonal planar polar-trigonal bipyramidal polar. Monoisotopic mass 115915863 Da. Contamination of BCl3 with COCl2 from the chlorination of carbon oxides is a serious problem in BCl3 manufacturing.
ANumber of central atoms. Linear Therefore the shape of the molecules are arranged so that the energy is minimized. What is the molecular geometry of BCl3.
E Number of lone pairs on central atom. Of monovalent atom are 3 three chlorine both above adds to give 6 and divided by 2 equals to 3 therefore hybridisation will be sp2 and shape will. See full answer below.
AX 3 has trigonal planar shape.

Bcl3 Lewis Structure And Molecular Geometry Youtube

Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube
What Are The Hybridisation And Shape Of Bcl3 Quora

Bcl3 Boron Trichloride Molecular Geometry Bond Angles And Electron Geometry Youtube
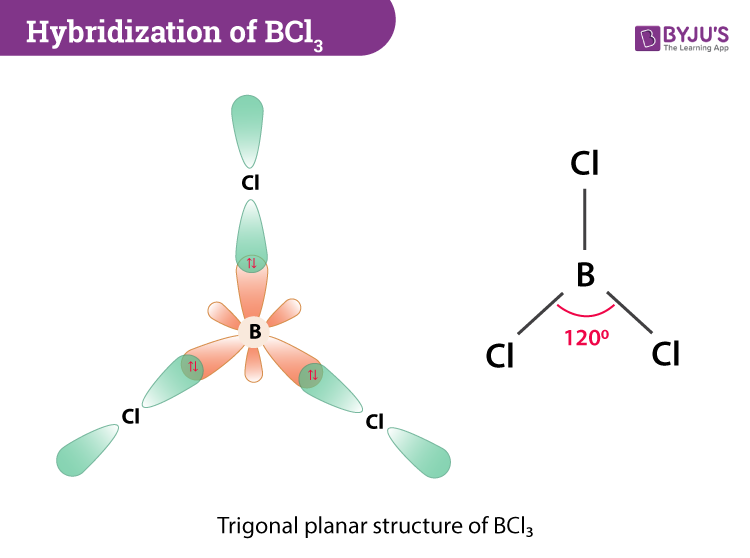
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
Bcl3 Lewis Structure Molecular Geometry Hybridization And Shape
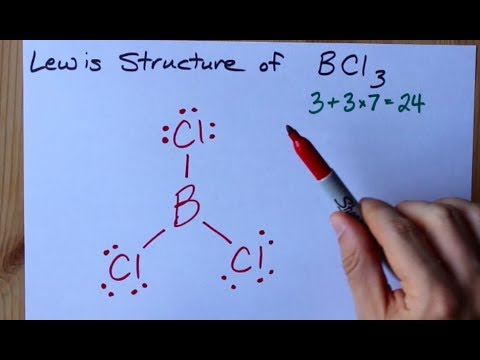
How To Draw The Lewis Structure Of Bcl3 Boron Trichloride Youtube
Post a Comment for "Bcl3 Molecular Shape"